OTCKing - Helping tomorrow's FORTUNE 500 companies TODAY!
Gala Global, GLAG, Profile,Summary

Gala Global, GLAG, Profile
GALA Global, Inc. is a publicly traded development stage company which plans to expand in the Hemp and Cannabis industries. The Company is refocusing its purpose on the development, research, and commercialization of products derived from Hemp and Cannabis Plant, while expanding the clothing operations and upcoming Hemp based fiber apparel and accessories division.
The Hemp Industries Association (HIA) estimated that the 2014 retail sales value of hemp food, body/beauty care, clothing and various other hemp products sold in the United States were more than $620 million.
Industrial Hemp is a crop that can be grown for food and non-food purposes. As a result of its numerous nutritional benefits, many new food products containing hemp seed and its oil are finding their way into the marketplace, including pasta, tortilla chips, salad dressings, snack products and frozen desserts. Hemp seed contains a complete protein and the seed oil is the richest source of essential fatty acids (Omega-6 and Omega-3) with the perfect ratio of 3:1. Hemp seed oil is also used in nutraceuticals and health care products.
As a fiber source, hemp is undergoing rapid growth as a natural fiber in everything from clothing and textiles to automotive composites. Although it is legal to import and sale industrial hemp products in the U.S., it is not yet legal to commercially grow industrial hemp in the U.S. The 2013 farm bill was signed into law in February 2014 and contained a hemp amendment, Sec. 7606 Legitimacy of Industrial Hemp Research, which allows states that have already legalized the crop to cultivate hemp within the parameters of state agriculture departments and research institutions.
In January of 2015, The Industrial Hemp Farming Act was introduced in both the House and Senate, H.R. 525 and S. 134 respectively. If passed, the bill would remove all federal restrictions on the cultivation of industrial hemp, and remove its classification as a Schedule 1 controlled substance.
Subsidiaries
Gala Global owns and operates several subsidiaries including, Cannabis Ventures Inc., Cannabis Ventures (Canada) and CBDLiving.
Cannabis Ventures, Inc.
Cannabis Ventures (CVI) was organized for the purpose of developing leading scientific processing to cultivate a bio-medical grade Cannabis plant featuring a higher concentration Cannabidiol (CBD) value with natural healing powers. The team along with expert consultants which have specific expertise in pharmaceutical sciences (medicinal chemistry of natural products) and herbal pharmacology. Cannabis Ventures plans to supply the medical marijuana market along with alternate medicinal supply for global market for highly enriched plant concentration of Cannabidiol.
For more information visit www.cannabisventuresinc.com
Cannabis Ventures (USA)
Cannabis Venture signed a management agreement with America's first approved dispensary, Compassionate Caregiver! Compassionate Caregiver based in California became the first dispensary to provide Medical Marijuana in the United States.
Cannabis Ventures (Canada)
The company's Canadian subsidiary filed application to be a Licensed Producer under the Medical Marijuana for Medical Purposes Regulations (MMPR) with Health Canada. Cannabis Ventures is currently in the process in establishing operations in British Columbia to service the Medical Marijuana market.
Cannabis Ventures (Canada) recently signed a turn key management contract with a company based in Ontario, Canada. Cannabis Venture will assist in designing, equipment procurement, installation and help the company obtain license from Health Canada.
CBDLivin
Gala Global created this special division to take advantage of $18 Billion dollar supplement industry. The company plans to introduce CBD infused dietary supplements and sports nutrition products in 23 states and the District of Columbia in the near future. The company believes Cannabidiols (CBD) Cannabinol ("CBN") could be a safer and better alternative for the treatment of sleep and anxiety disorders. 50 million Americans suffer from sleep and anxiety disorders, a large number of them use Prescription and/or OTC (over-the-counter) drugs, some of these drugs are habit forming, many Americans are unfortunately becoming drug-dependent and addicted to sleeping pills and pain killers and authorities are very concerned about the adverse social and economic consequences of the addiction problem.
For more information visit www.cbdlivin.com www.galaglobalinc.com
Sources: The Company, OxBridge Research, OTCKING, DailyStockDeals, OTCstockIQ
Don't miss the NEXT premium Alert! Sign-up, Get Alerts, MakeMoney!®
Disclaimer/Disclosure: we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks.
If you would like your company featured or want to learn more, please don't hesitate to contact the Editor. editor [@] DailyStockDeals.com
Tanaris Power, TPHX, Profile, Summary

Tanaris Power, TPHX, Profile, Summary
Tanaris Power develops Smart Lithium-Ion batteries and power solutions for various industrial applications. We design, engineer and produce some of the worlds safest, highest performance, most reliable and flexible Lithium-Ion batteries.
Tanaris Power lithium-ion batteries feature best-In-class safety, reliability, performance and flexibility for your industrial power applications
All Tanaris Power battery packs are based on Lithium-ion battery technology. Each of our batteries incorporates our advanced Battery Management System (BMS) along with numerous redundant safety and performance features to ensure that our batteries are the safest, most reliable, best performing and flexible batteries available
Tanaris Power Lithium-Ion Benefits
Our lithium-ion batteries offer numerous benefits over conventional lead-acid batteries, including:
-
Significantly improved vehicle performance
-
Significantly longer run time between charges
-
Short duration charging has no negative effects
-
3x to 5x longer life span than lead-acid batteries
-
65-70% less weight than similar lead-acid packs
-
Completely maintenance free operation
-
No acid vapor, maintenance or storage risks
-
Integrated BMS for system monitoring and safety
-
Ruggedized design for extreme outdoor use
Safety
-
Your safety is at the core of every design decision and component selection we make
-
All of the Lithium-Ion cells we use must meet international UN38.3 standards for safe transportation and usage
-
We use the Lithium-Iron-Phosphate chemistry for our cells, which provides a level of thermal stability that some other cell chemistries do not offer
-
Every battery we make incorporates a dedicated advanced Battery Management System (BMS) along with other redundant intelligent monitoring circuitry that monitors each individual cells' operation and temperature to ensure that the whole pack operates safely
-
Each and every battery incorporates numerous additional safety features such as an integrated battery isolation fault monitoring system, redundant fuses, sealed safety relays, audible alarms and much more
Reliability
-
We only use the best quality automotive grade components to ensure maximum reliability
-
Each and every one of our batteries and the components that go into them, go through extensive burn-in and testing processes before they make it into any Tanaris Power battery
-
Every batch of Lithium-Ion cells are subjected to an array of standardized tests to ensure they are well qualified before they go into any battery
-
All cells are are secured using specialized hold-down mechanisms within the battery to reduce shock and vibration
-
BMS and all electronics are designed to strict automotive standards, using only automotive and industrial grade components, for the highest level of reliability in all operating environments
-
All battery cases and electronic connectors are sealed to ensure the battery will work in all weather conditions
Performance
-
Rigorous in-house cell testing and characterization allows us to manage and optimize the cell selection for each battery application, ensuring optimal power delivery for the life of the system
-
We test and evaluate cells from a wide variety of manufacturers to find the best performing cells
-
Our advanced Battery Management System maintains peak performance of all cells in real time
-
Proprietary cell balancing algorithms ensure that all battery cells maintain their balance at all times
-
All aspects of battery charging are under real time monitoring and direct control of the BMS which means the BMS selects the appropriate charging profile in order to extend battery life
-
Cell behavior is learned with usage, which allows the system to better predict vehicle demands and understand overall battery health flexibility
-
The BMS calculates and compares cell-by-cell performance to ensure peak battery performance
Flexibility
-
Battery packs are available in industry standard voltage levels such as 24V, 36V, 48V, 72V and 80V
-
Battery capacity can be selected to match application requirements at each voltage level, from 40Ah to 900Ah
-
Single or multi-module battery pack configurations are available depending on specific space or layout requirements
-
Physical dimensions of many battery modules can be customized to client specific needs
-
Integrated CAN bus communications can be customized to interface with other intelligent equipment such as on-board chargers, charging stations, motor controllers, driver displays, etc.
-
BMS control logic can be customized to interact with and control other vehicle equipment such as emergency stop button, key-switches, analog or digital gauges, mode selection switches, buzzers, sirens, lights and more
Sources: The Company OxBridge Research, OTCKING, DailyStockDeals, OTCstockIQ
Don't miss the NEXT premium Alert! Sign-up, Get Alerts, MakeMoney!®
Disclaimer/Disclosure: we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks.
If you would like your company featured or want to learn more, please don't hesitate to contact the Editor. editor [@] DailyStockDeals.com
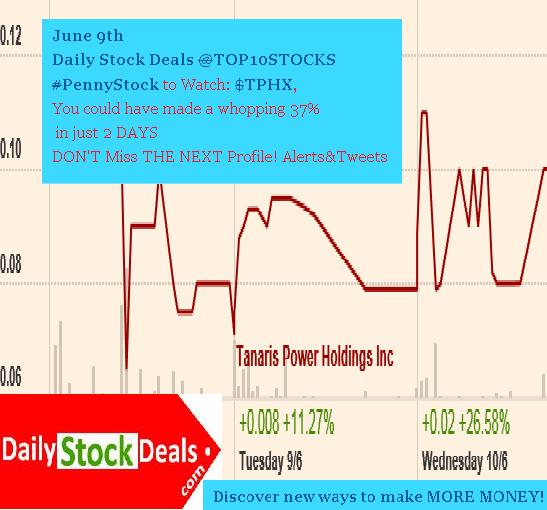
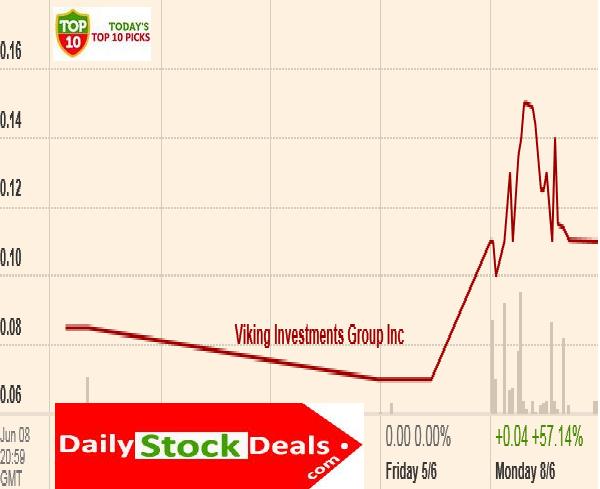
Viking investments,VKIN,Profile,Summary

Viking Investments Group, VKIN, Profile, Summary
Viking Investments Group provides professional advisory and consulting services to companies undergoing or anticipating periods of rapid growth, significant change or ownership transition, and when justified, staffing, financing, and/or providing operational support to such companies. Target companies must have superior management, intimate knowledge of their particular industry and a sound business plan, along with a desire and receptiveness for specific expertise to advance the company's business objectives.
Viking’s primary focus is directed toward North America, targeting various industries,mainly in the Oil & Gas sector and other selective sectors, with appropriate diversification and balance between each. Viking targets under-valued businesses with realistic appreciation potential and a defined exit strategy.
Current Projects
The Joffre D-3 Oil Project is located in the Leduc D-3 B Pinnacle Reef in Central Alberta, Canada (the “Joffre Project”).
Viking owns a 50% working interest in the Joffre Project which consists of 4 oil wells and one water injection well. The wells were previously suspended. Viking and Tanager Energy Inc., Viking’s partner for this project, mobilized the first well (production commenced on April 1st, 2015), and intend to mobilize the remaining wells sequentially.
After 5 days of anticipated monitoring and operating facility adjustments, the well is now producing at a rate of 118 barrels of oil per day of 38 API gravity crude oil and 250 mcf of natural gas, totaling 160 BOE/D. The well is a flowing oil well with a flowing wellhead pressure of 435 psi.
Viking estimates to complete the re-work of the remaining wells during the second and third quarter of 2015.
Mobilization and reactivation efforts involve, among other things, completing the re-entry and downhole pressure survey to determine the current reservoir pressure, a critical element in determining the scope and anticipated production of this suspended oil pool. The survey with respect to the first well confirmed the reservoir pressure returned to 94% of the original pressure (i.e. the pressure that existed when the pool was discovered in 1986) due to the active water drive in the pool.
Following the pressure test described above, a production test is performed to confirm the productivity of the pool. The production test for the first well occurred between January 13th and January 15th, 2015 and the test results indicated the following:
-
a stabilized production rate of 290 BOE/d (235 barrels per day of oil and 325 mcf/d of gas).
-
the flowing wellhead pressure was stable at 475 psi, estimated to be a 7.7% drawdown of the reservoir. On a metric equivalent basis this is 52.5 cubic meters oil equivalent per day at 3265 kpa flowing wellhead pressure.
-
The fluid produced was 100 percent high gravity oil.
Sources: The Company, OxBridge Research, OTCKING, DailyStockDeals, OTCstockIQ
Don't miss the NEXT premium Alert! Sign-up, Get Alerts, MakeMoney!®
Disclaimer/Disclosure: we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks.
If you would like your company featured or want to learn more, please don't hesitate to contact the Editor. editor [@] DailyStockDeals.com
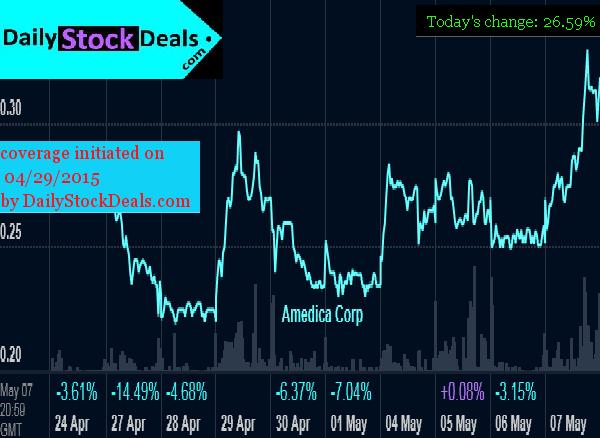
Amedica Corporation, AMDA, Profile, Summary
Amedica Corporation, AMDA, Profile, Summary

Amedica Corporation, AMDA, is a commercial biomaterial company. The Company is focused on using its silicon nitride technology platform to develop, manufacture and sell a range of medical devices. The Company markets spinal fusion products and are developing products for use in total hip and knee joint replacements.
Biomaterials are synthetic or natural materials available in a variety of forms that are used in virtually every medical specialty. The Company markets its Valeo family of silicon nitride interbody spinal fusion devices in the United States and Europe for use in the cervical and thoracolumbar areas of the spine. In addition, to its silicon nitride-based spinal fusion products, it markets a complementary line of non-silicon nitride spinal fusion products which allows providing surgeons and hospitals with a range of products. The Company produces its silicon nitride advanced ceramic in four forms: a fully dense, load-bearing solid, referred to as MC2; a porous bone-like cancellous structured form, referred to as CSC; a composite incorporating both its solid MC2 material and its porous CSC material intended to promote an environment for bone growth; and a coating for application onto other biomaterials.
The Company competes with Medtronic, Inc.; DePuy Synthes Companies, a group of Johnson & Johnson companies; Stryker Corporation; Biomet, Inc.; Zimmer Holdings, Inc.; Smith & Nephew plc; Aesculap Inc; CeramTec, Kyocera, and CoorTek, Inc.
OSTEOPROMOTIVE: DELIVERS ENHANCED OSTEOGENIC RESPONSE
-
Enhanced osteopromotion: The surface chemistry and nanostructure topography of Si3N4 provides an optimal environment for stimulation of osteoprogenitor cells to differentiate into osteoblasts.
-
Greater protein adsorption: Si3N4 demonstrates significantly greater protein adsorption (fibronectin, laminin and vitronectin) in comparison to PEEK and titanium. (1)
-
Greater new bone formation: Si3N4 implants demonstrate greater new bone formation at 3, 7, 14 and 90 days compared to PEEK and titanium in in vivo study; regenerated bone associated with Si3N4 implants is 2 to 3 times that of PEEK and titanium implants at 3 months after surgery.(2)
-
Increased osteointegration: Si3N4 implants demonstrate increased osteointegration at 3, 7, 14 and 90 days compared to PEEK and titanium in in vivo studies; percent of bone at Si3N4 implant interface is 2 to 6 times that of PEEK and titanium implants at 3 months after surgery.(2)
DEMONSTRATED ANTI-INFECTIVE PROPERTIES
-
Superior antibacterial function: Si3N4 inhibits biofilm formation and bacterial colonization. Si3N4 demonstrates significantly lower biofilm formation at 4, 24, 48 and 72 hours as compared to PEEK and titanium; live bacteria (S. epidermidis, S. aureus, P. aeruginosa, E. coli and Enterococcus) associated with Si3N4 implants are 8 to 30 times lower than PEEK and titanium.(1)
-
Demonstrated bacteriostatic agent: In in vivo studies, no infection is observed with bacteria-inoculated Si3N4 implants at 3 months, whereas both PEEK and titanium implants maintain a septic state. Si3N4 demonstrates this property even in the absence of antibiotics.(2)
EXCELLENT IMAGING PROPERTIES
-
Compatible with all imaging modalities: Si3N4 implants are semi-radiolucent with clearly visible boundaries, and produce no distortion under MRI and no scattering under CT; this enables an exact view of the implant for precise intraoperative placement and postoperative fusion assessment.
Sources: The Company, OxBridge Research, OTCKING, DailyStockDeals, OTCstockIQ
Don't miss the NEXT premium Alert! Sign-up, Get Alerts, MakeMoney!®
Disclaimer/Disclosure: we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks.
If you would like your company featured or want to learn more, please don't hesitate to contact the Editor. editor [@] OxBridgeResearch.com

 OTCKing
OTCKing Feed Entries
Feed Entries
